Another aspect of a coolant’s chemistry is hardness, measuring the amount of minerals present.
Hardness is one of the least-appreciated and most-overlooked aspects of coolant chemistry. Previously, you learned that coolant concentrate can be mixed with water in a 1:20 ratio. The water supply contains minerals such as calcium (Ca++) and magnesium (Mg++) that we need to pay attention to.
Initial Water Hardness vs Effects of Evaporation
Most often the initial water supply is within the hardness range that a coolant can chemically withstand. However, when evaporation happens, only water (H20) is lost, leaving minerals behind. Over time, these minerals build up in the sump and wreak havoc on the coolant emulsion, or mixture. This is because these minerals have a high positive charge, whereas coolant has a high negative charge. And, as they say, opposites attract!
Effects of Hardness on the Coolant Emulsion
What happens when this attraction takes place? Look at the appearance of the coolant samples in the illustration below. The left beaker contains a sample of the coolant when it is first mixed and has a good oil-in-water emulsion. The coolant mixture is stable since the initial water source measures 180 ppm while the coolant can withstand up to 200 ppm.
After five refills of this sump, using the same water supply, the hardness level has increased to 220 ppm. At this point the middle beaker looks similar to spoiled cream in coffee. After ten refills, the right beaker has a mineral hardness of 280 ppm which has ‘broken’ the oil-in-water emulsion. The coolant concentrate ‘splits’ from the emulsion and floats to the surface.
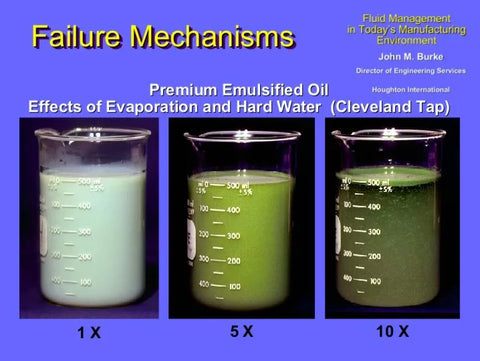
When this happens in a sump, the pump pulls mostly water from below the fluid surface, since the emulsion has split. Imagine how this affects the machining process: almost all lubrication is lost. Tooling breaks easily, surface finishes are rough, and valuable production time and material are lost.
Since the coolant concentrate is composed of oils, and its oil component is floating, it can also be removed by mechanical skimmers and sent to waste. This heavily concentrated oil also plugs particle filters within coalescers, and in some cases, particle filters for the machine pump.
Water is not the same everywhere. We seldom think about it, but ground water in one part of a state may be very different from ground water in another. Even city water is not the same across the country. For instance, there are regions in the US that have water with high hardness levels about 200 ppm. These are regions where extra caution must be taken.
Although the initial water supply may not affect the coolant emulsion, the buildup of minerals after evaporation can render the coolant useless.
Should water softener systems not be sufficient to remove these dangerous minerals, then consider de-ionized or reverse osmosis water filtration systems. These specialty systems are an investment, but usually much less expensive than the alternative.
Conversely, some areas lack minerals in the water causing coolant to foam, which could make the sump overflow and inhibit proper tramp oil removal. Many coolant suppliers offer additives which reduce foam by adjusting the hardness level so that it is within appropriate limits.
